With new legislation forthcoming, firms need to start preparing now and ensure they and their supply chain understand compliance requirements

Product safety is a major concern, with the influx of cheaper imports into the European markets. This necessitates a constant survey of markets and scrutinising of products for the risks they might pose to consumers. In a bid to survive and remain competitive, EU industries are trying to enhance the quality of their products. However, they are beleaguered by different legislations and market surveillance systems.
To do away with the individual national regulations, the European Commission (EC) is proposing a homogeneous set of directives to simplify and harmonise consumer product safety and market surveillance in the EU. Although this legislation has not been finalised yet, organisations need to gear up for upcoming regulatory changes and start preparing now.
Current legislation and rules
- Directive 2001/95/EC on General Product Safety maintains the basic safety provisions with which all consumer products must comply (except for food and medical devices). Member states (MS) should ensure that: (i) products meet the applicable safety requirements; (ii) steps are taken to make products compliant; and (iii) sanctions are applied where necessary.
- The new Approach Directive deals with specific categories of products.Its terms are wider and incorporate technical standards to specify details for each product that falls within its framework. It includes safeguard clauses for products that may be liable to compromise the health and safety of persons, specifying that it must then be recalled from the market.
- Regulation (EC) 765/2008 on accreditation and market surveillance is related to the marketing of products and sets the procedures required to “establish, implement, and update national market surveillance programmes”. It is important to distinguish between the harmonised and non-harmonised products. The former comprise products, such as lawn mowers, lifts and pressure equipment regulated by EU legislations.
- Regulation 764/2008 deals with non-harmonised products, such as handbags, wooden articles and wood or toothbrushes, for instance, for which the rules have not been uniformly adopted in the EU and may vary from one MS to another. The regulation ensures that these non-harmonised products can be freely sold in all MSs on the basis of the mutual recognition procedure principle.
- The Rapid Alert Information System (RAPEX) enables the “rapid exchange of information between MSs and the EC on measures taken to prevent or restrict the marketing or use of products posing serious risks to the health and safety of consumers (with the exception of food and medical devices which are covered by other mechanisms)”.
Proposed changes
In 2013, the EC adopted a Product Safety and Market Surveillance Package. This proposes a new regulation on: (i) consumer product safety to merge all safety rules for consumer products into a single legislation (not applicable to food and medicinal products); (ii) market surveillance for products; (iii) and a multi-annual action plan to improve market surveillance rules in the next three years. If approved, the Draft Regulation on Consumer Product Safety would create a new framework for the market surveillance of products.
Although the scope of the proposed regulation is expected to be the same, it will, however, be further clarified. It will not apply to medical devices and building materials and will apply to the harmonised and non-harmonised categories of products.
These changes are expected to simplify and make uniform the legislative and non-legislative measures, with regard to safety guidelines for non-food products distributed in the EU. In addition, these will also bring about common standards in market surveillance procedures, their co-ordination and monitoring, consumer product identification and tracking, enforcement of product safety rules, while placing additional responsibilities on stakeholders, manufacturers, importers and distributors whose products are sold in the EU.
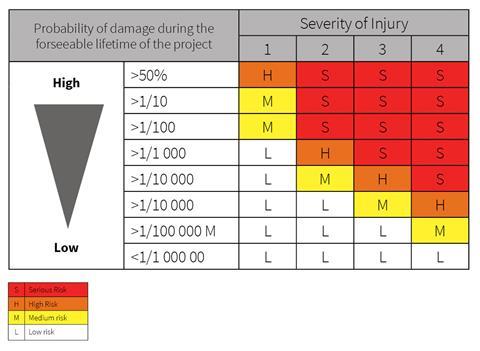
- Safety assessments by product categories: under the proposed changes, safety assessments will differ according to the category of the product. For those products covered by the harmonised legislation, risk assessments will be made on the basis of the EU harmonised legislation or the national laws of the MSs or European standards. For those products not covered by harmonised legislation, risk assessments will be based on the existing national legislations, if any, or on the basis of criteria included in Directive 2001/95 (GPSD).
These proposed changes also set the responsibilities of the various stakeholders – the economic operators – which will need to guarantee the safety of the product via comprehensive documentation, list the risks posed and adopt effective solutions to mitigate these.
In addition to setting the different levels of obligations between the different economic operators, these changes may apply exceptionally to commercial products, online sales and confirm the application of the precautionary principle.
- EU safety tested (CE plus): a new provision called the CE Plus has been included by the European Parliament (EP) by which there should be a difference in marking for those products testified by certified labs (CE Plus), as opposed to those tested by companies internally (CE). Although some MSs rejected this, it may be reintroduced in the future.
- Indication of origin: although supported by the EP, this provision is a point of contention between northern and southern MSs. While the indication of a country of origin of a product has been considerably debated following pressure by Italy and France, the EC had to introduce this aspect. Under this principle, manufacturers and importers would have to include the name of the country of origin on their products. However, if the size and nature of the product did not allow for the name to be included, this would have to be inserted on all documents accompanying the product. This provision is essential because it compliments traceability and enables consumers to easily access information on the product chain.
- Traceability: the EC may require economic operators to establish or adhere to a system of traceability for certain products, categories or groups of products that could bear a serious risk to health and safety of consumers owing to characteristics or conditions of usage and distribution.
- Emerging risk: a product about which scientific evidence shows that it presents a new or a known risk if used in new or unusual conditions that cannot be reasonably foreseen by the manufacturer is defined as emerging risk. Here, the EC can set out uniform conditions to conduct checks by reference to particular product categories or sectors.
- Streamlined corrective actions: the six hypothetical types of measures the economic operators are required to undertake under the GPSD have been reduced to four: (i) non-compliant product; (ii) product liable to present a risk only in certain conditions or only to certain persons; (iii) product that may present a serious risk; and (iv) product that presents a serious risk.
- RAPEX is extended to the notification of products presenting a risk (not only those products that bear a “serious” risk). Punitive action may be taken against those not conforming to the set of rules spans the application of sanctions, blacklisting and imposition of fines.
How to ensure compliance
The EU product safety requirements have not been finalised yet, but firms need to start preparing, keeping the many sanctions and principles in mind. Since compliance, product safety and surveillance go beyond the individual companies and extend to relationships with third parties, technology will become an enabling part of all compliance-related activities.
The first step is to understand compliance requirements and draft compliance-related rules affecting the organisation and those parts of its supply chain. It then becomes easier to adapt technology to the organisation’s compliance needs. Technology can then become the backbone of all organisational and compliance-related tasks.
Below are a few key areas where technology can help:
- Information management: information management systems can help capture and map relevant suppliers, sub-suppliers, products, and third-party lab information in a centralised repository. A robust information management system can help manage relationships between supplier, product, material, attribute and compliance. It will provide complete visibility across the supply chain and ensure traceability.
- Policy management: companies can leverage technology to create, communicate and manage policies across the organisation and supply chain. Industry best policy management systems are equipped with sophisticated collaboration and workflow tools that can be used to access, create, modify, review and approve policy and procedure documents globally in a controlled manner. A centralised, web-based repository helps store and organise policy and procedure documents, map policies to compliance requirements, and provides easy search and access capabilities.
- Risk management: an advanced risk management tool enables organisations to design and conduct risk assessments to identify and mitigate product quality risks. Powerful risk heat maps and risk calculators will help assess, rate and prioritise risks such as non-compliance risks and consumer safety hazard risks.
- Product testing: technology systems can provide a single framework to create product test plans and manage each stage of testing. These systems can also facilitate integration with testing labs to import findings and results across the entire testing lifecycle. A centralised database makes it easy to manage testing information on product properties, specifications, attributes and regulatory compliance.
- Inspection management: technology can be adopted to automate inspection planning, sampling plans and inspection criteria. These solutions provide flexible means to manage inspection information, sample information, inspections criteria, scheduling of inspections, templates, questionnaires and workflows to manage inspections.
- Audit management: audit management systems can be used to manage internal quality audits, supplier audits and external or thirdparty audits and inspections to determine if quality and compliance requirements are being followed. Advanced inbuilt capabilities can help plan and schedule the audits, conduct fieldwork based on audit checklists, review and analyse audit findings and initiate follow-up activities.
- NCM & CAPA: a sophisticated NCM & CAPA management system can be adopted to ensure centralised management and tracking of all issues and corrective/preventive actions as well as product recalls. Through automatic rule-based routing, non-conformance issues can be sent to the appropriate teams for review and disposition. These solutions have inbuilt capabilities to support issue investigation, root cause analysis, assignment of follow-up actions and initiation of corrective and preventive action.
By leveraging these areas, it is possible to streamline, integrate and automate many product safety and compliance processes spanning audits, inspections, risk assessments, product testing and preventive and curative actions. Such systems enable real-time communication and training while improving quality and safety compliance across the enterprise and supply chain, thereby offering better visibility leading to building better brands.
Further, these solutions bring all the stakeholders together on a common platform, streamlines compliance with regulations pertinent to a company’s upstream and downstream processes, while tracking the product through its lifecycle for compliance with high standards of quality.
Organisations can also strengthen their compliance efforts by systematically developing reporting capabilities, filtering, storing, retrieving data when required, data crunching, appropriate data usage, and backing up and archiving current data.
Preparing for compliance
To guarantee product safety and effective market surveillance and to enable their seamless functioning across global markets, organisations need to be prepared for new and evolving compliance requirements. This also requires that management assumes greater ownership over risks, especially as stakeholders become more vocal about organisations failures and mis-steps. It is vital to understand all the proposed regulatory changes, design effective product safety compliance programmes and leverage technology to strengthen these programmes.
Jean-Luc Laffineur is a partner at Laffineur Law Firm and Cédric Merahi is a risk management specialist at MetricStream
Downloads
PDF of article
PDF, Size 0.65 mb



















No comments yet